Dr Tara M Finegan
MPhil MA(Cantab) PhD
Assistant Research Professor
Division of Biological Sciences
University of Missouri
Trans-disciplinary approaches to tissue architecture in development and disease.
This is my personal research site. View our main research group website here.
Research Summary
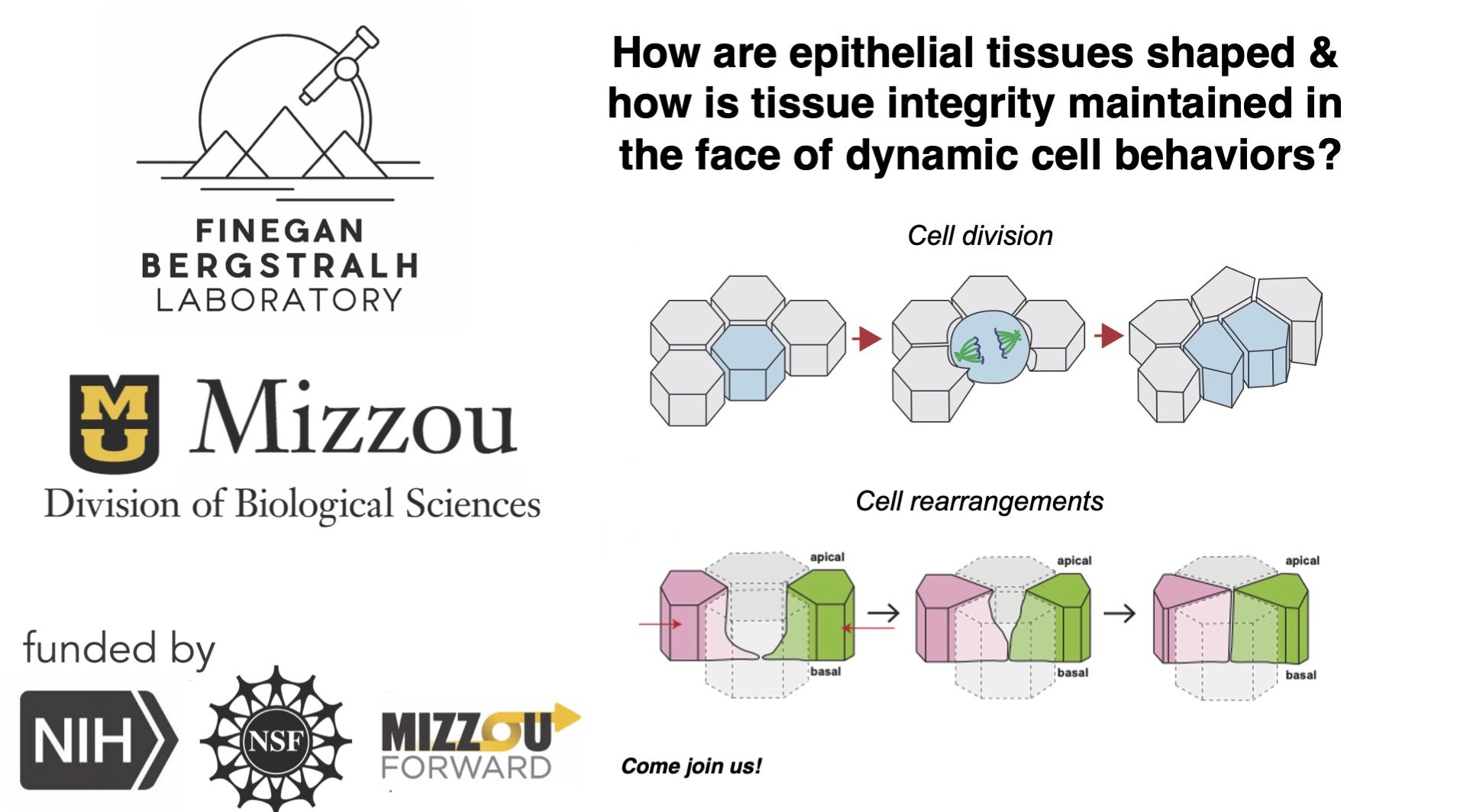
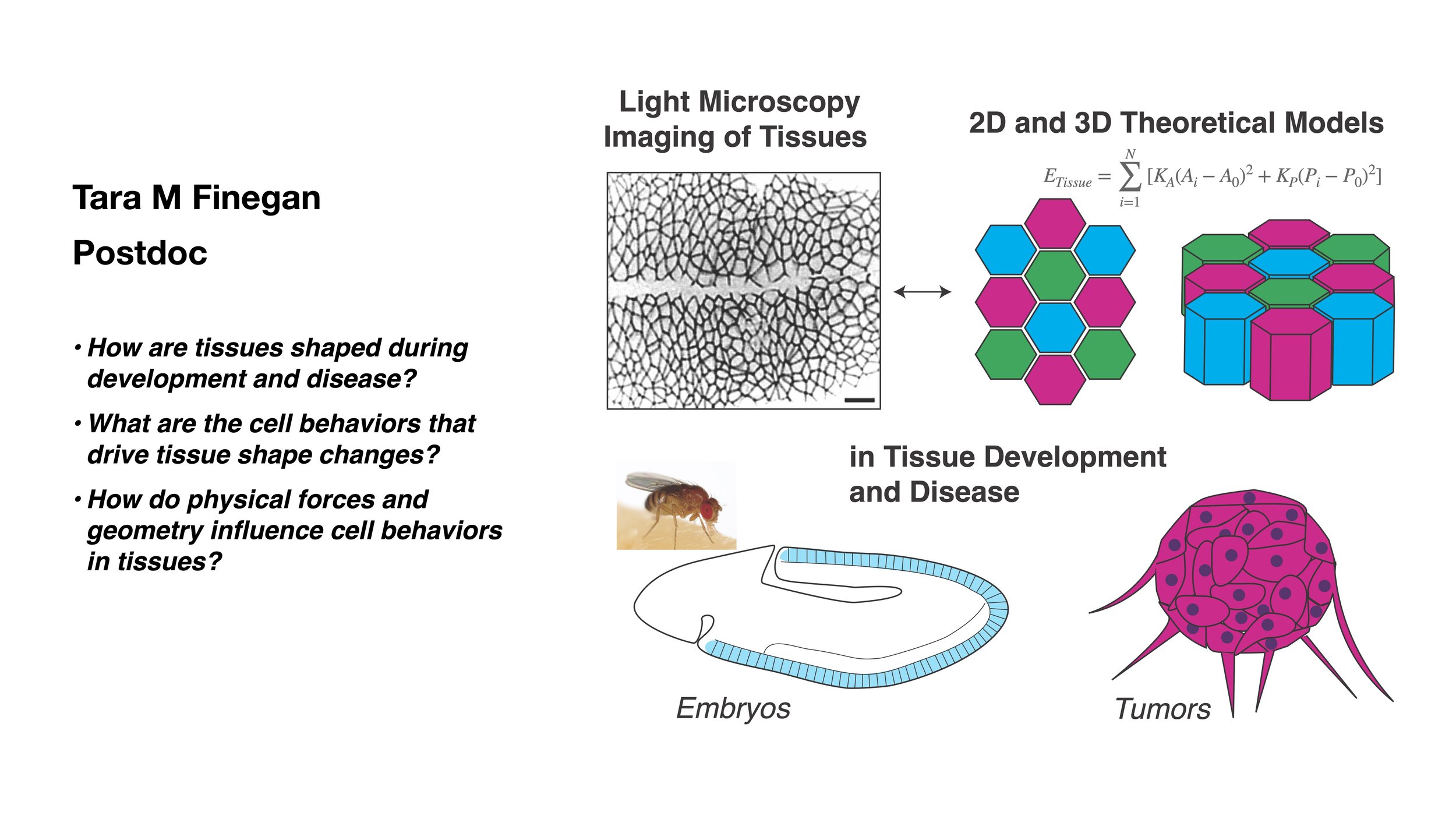
I am interested in how biological tissues interact with their physical environment and how the shape and material properties of tissues and their composite cells determine their behavior. These basic questions are important for understanding animal physiology, development and disease. I work at the interface of several scientific disciplines: biology, physics, and materials science.
I am broadly interested in how animal tissues acquire their shape, and what happens when that shape goes wrong. My work uses observation and modeling of the 3D structure, shape and dynamics of tissues using advanced light microscopy, quantitative analysis, theory and simulations. I am particularly interested in how members of the Immunoglobulin Cell Adhesion Molecule Family (IgCAMs) regulate cell shape and tissue morphogenesis.
I currently run a joint lab with my co-PI with Dan Bergstralh. We study the behavior and shape of animal epithelial tissue monolayers using Drosophila and cultured mammalian cells as our model systems. We are particularly interested in the dynamics and shape of cells in the relatively less-well understood apical-basal (depth) axis of epithelial tissues. Our main lab website is here.
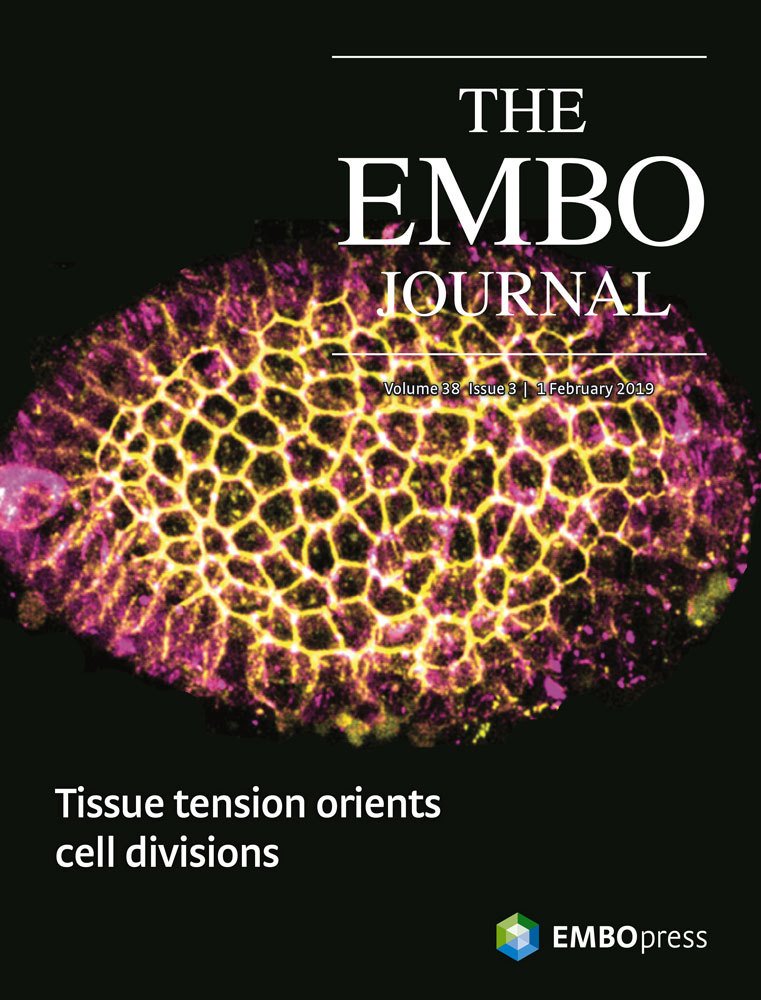
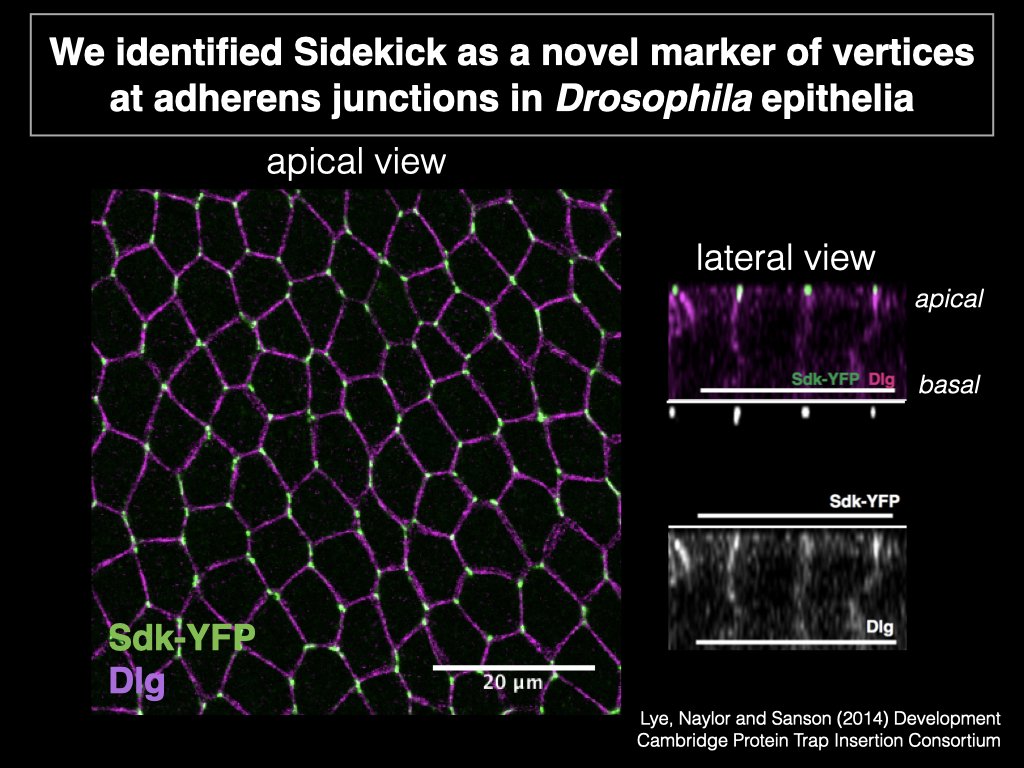
I obtained my PhD from the University of Cambridge in 2018 in the lab of Bénédicte Sanson as part of the Wellcome Trust Developmental Mechanisms PhD program. As a graduate student I identified the IgCAM Sidekick as a novel, conserved regulator of epithelial cell rearrangements in Drosophila (Finegan & Hervieux, et al. 2019, PLoS Biology). I continue to work on how adhesion proteins regulate the shape and dynamics of the germband in Drosophila embryos in collaboration with the lab of Mark Peifer at the University of North Carolina. As a post-doc at the University of Rochester I discovered that cells in a somatic ovarian epithelial tissue break a fundamental law of cell biology (Hertwig’s law, 1884): their shape does not determine their division orientation. Instead, the orientation of cell division is determined by tissue-level tension transmitted through apical junctional complexes (Finegan et al. 2019, EMBO Journal). Related to this work, I have also addressed how the mitotic spindle orienting machinery is localized and activated such that daughter cells are born in the correct direction in the context of an epithelial tissue (Neville & Finegan et al. 2023, EMBO Reports).
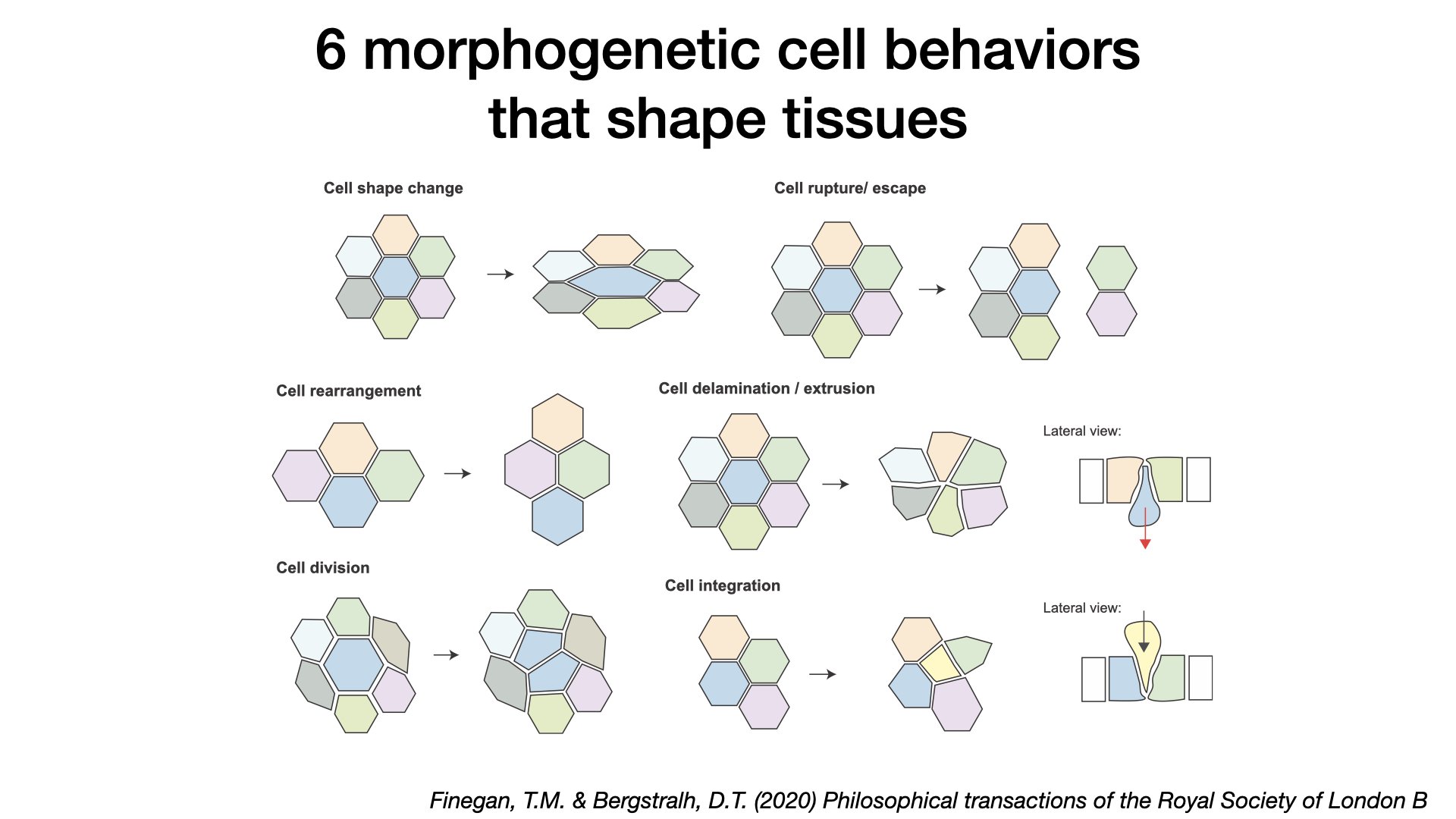
Our lab has demonstrated a mechanistic link between IgCAM proteins that line epithelial cell-cell borders and proteins that drive axon guidance in the developing nervous system (Cammarota & Finegan et al. 2020, Finegan & Bergstralh et al. 2020, Philosophical Transactions of the Royal Society B). We are now working on further elucidating the mechanism of how this machinery drives misplaced cells into epithelial monolayers, and if this machinery is conserved in vertebrates.